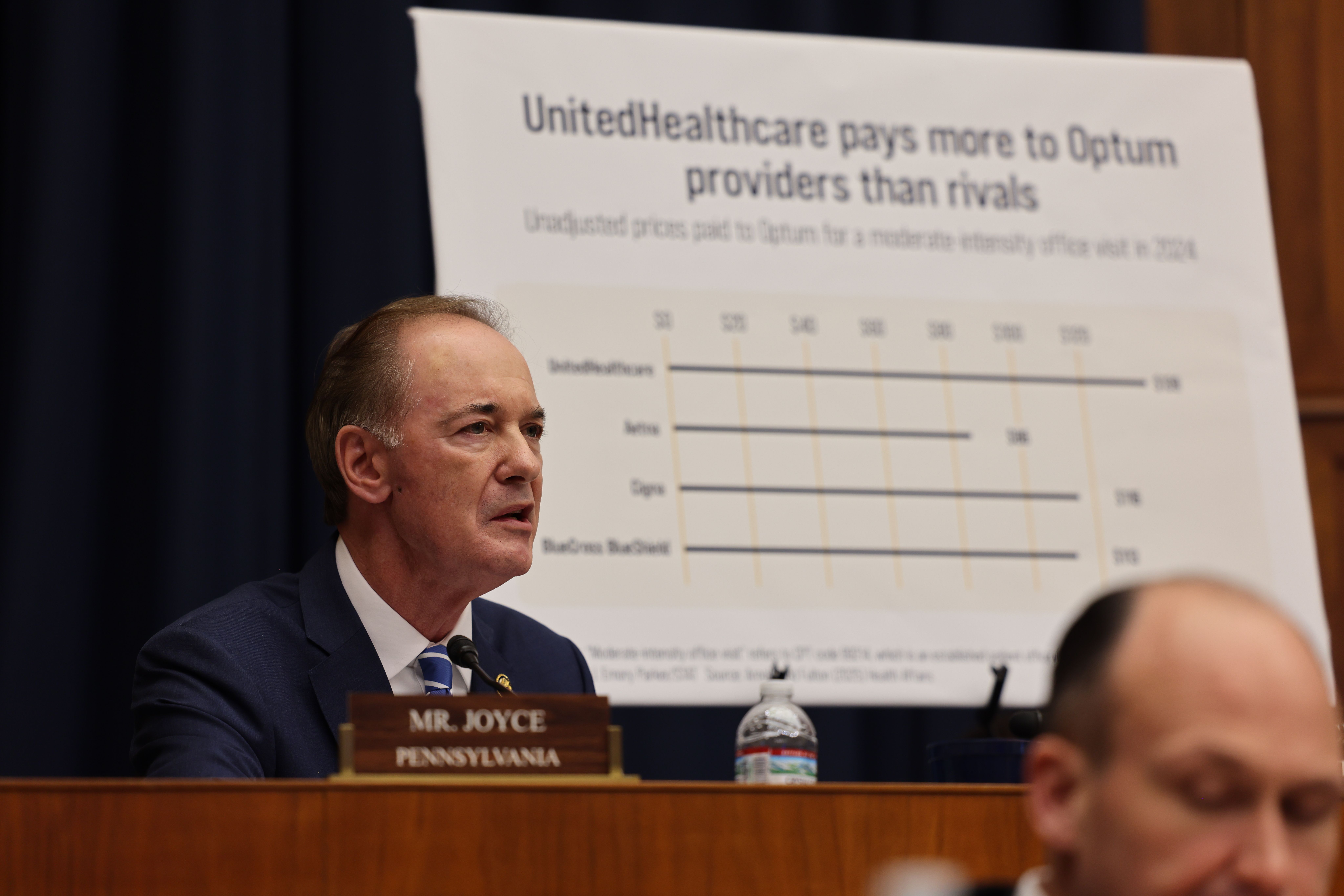
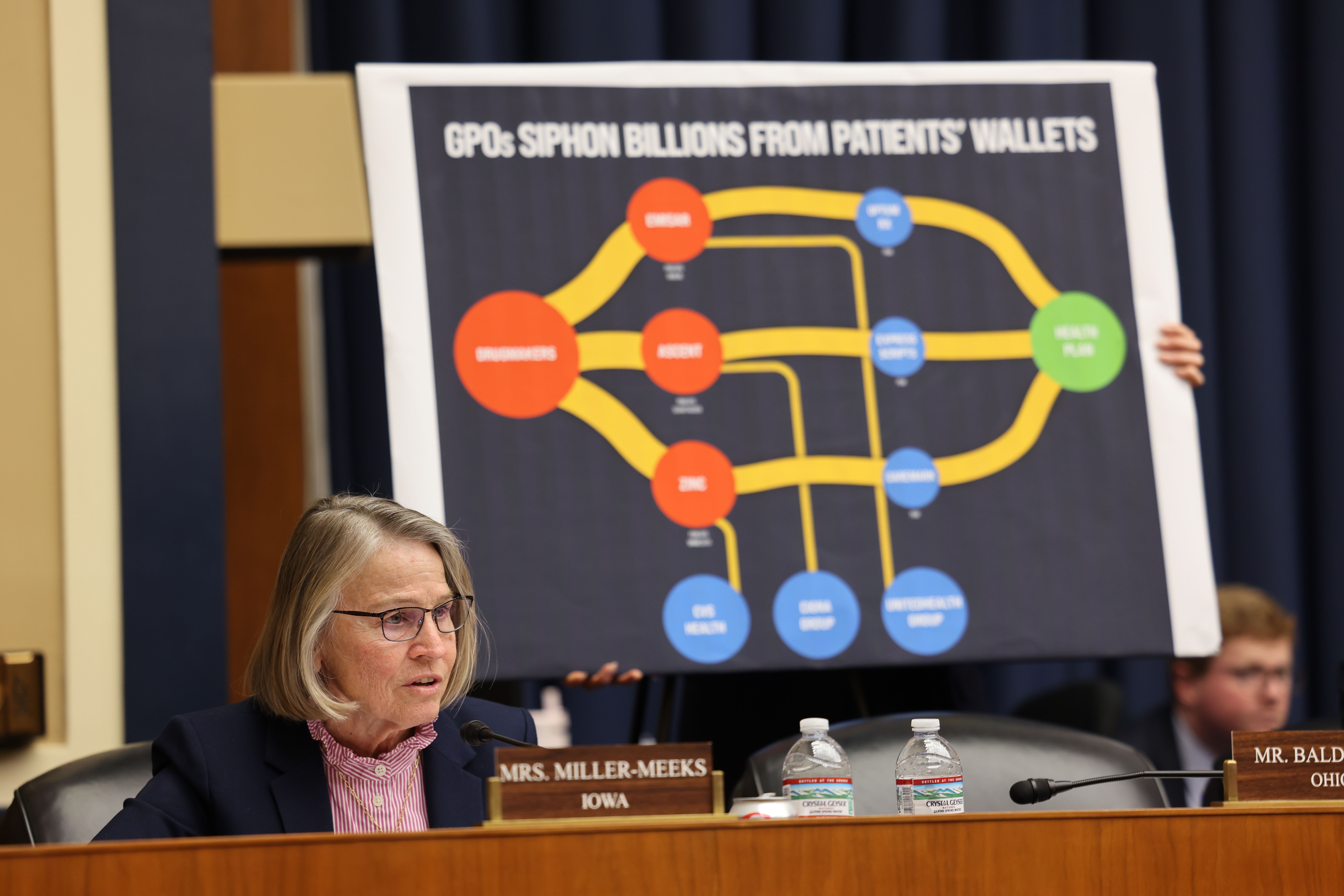
Chairmen Guthrie and Hudson Announce Legislative Hearing on FirstNet Reauthorization
WASHINGTON, D.C. – Today, Congressman Brett Guthrie (KY-02), Chairman of the House Committee on Energy and Commerce, and Congressman Richard Hudson (NC-09), Chairman of the Subcommittee on Communications and Technology, announced a hearing titled Evaluating FirstNet: Performance, Accountability, and Reauthorization.
“Starting out as a 9/11 Commission recommendation to strengthen emergency communications infrastructure, FirstNet has been a critical resource for first responders operating in moments of crisis,” said Chairmen Guthrie and Hudson. “As we continue to prepare for future man-made or natural disasters, FirstNet must remain on the leading edge of safety and reliability. We believe this reauthorization is a critical opportunity to increase transparency and effectiveness, so the program can fully achieve the intended goals that were originally conceived of two decades ago.”
Subcommittee on Communications and Technology hearing titled Evaluating FirstNet: Performance, Accountability, and Reauthorization.
WHAT: Subcommittee on Communications and Technology legislative hearing on FirstNet reauthorization.
DATE: Wednesday, February 4, 2026
TIME: 10:15 AM ET
LOCATION: 2123 Rayburn House Office Building
This hearing will focus on the following bill:
- H.R. ____, The First Responder Network Authority Reauthorization Act (Reps. Dunn and McClellan)
This notice is at the direction of the Chairman. The hearing will be open to the public and press and will be livestreamed online at energycommerce.house.gov. If you have any questions concerning this hearing, please contact Noah Jackson at Noah.Jackson@mail.house.gov. If you have any press-related questions, please contact Daniel Kelly at Daniel.Kelly@mail.house.gov.